Description
Package Includes
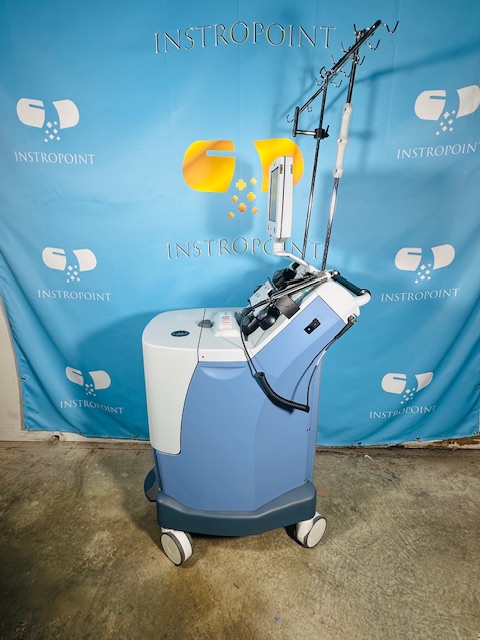
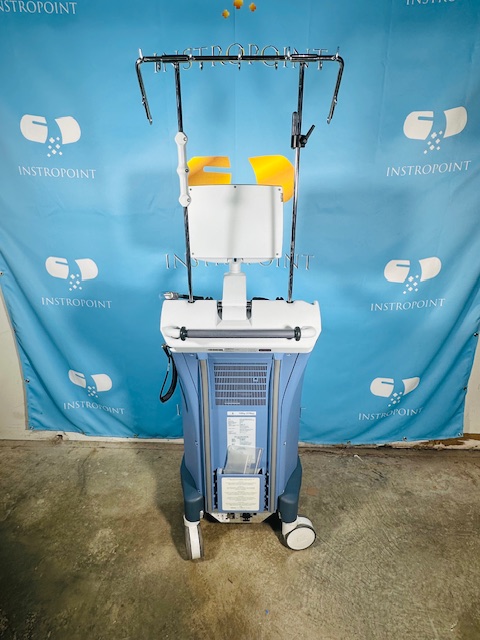
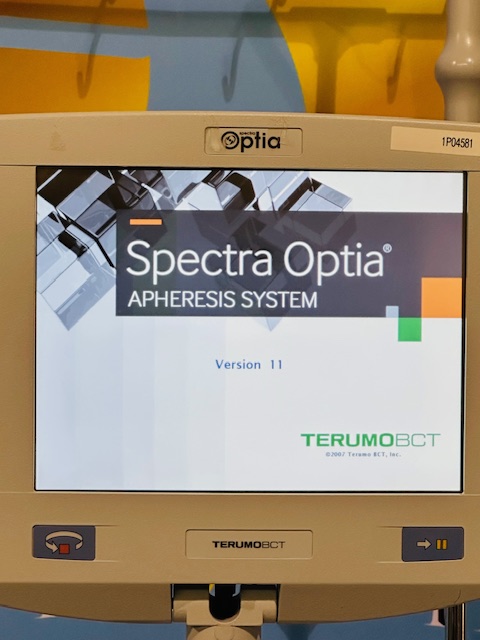
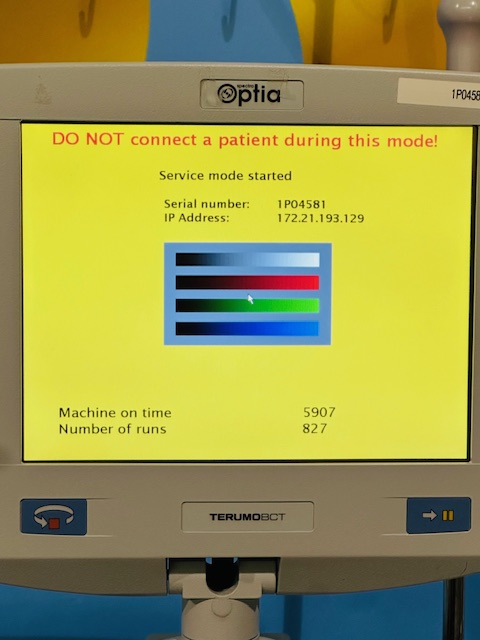
Everything shown in the photos is included.
If it’s not in the pictures and not listed here, it is not part of the sale.
If you have any questions about what’s included, please contact us before purchasing.
Item Condition
-
Unless specifically stated, full functionality has not been tested.
-
All photos are of the actual item — what you see is what you get.
-
This equipment must be checked and calibrated by qualified personnel prior to patient use.
-
As with most used medical equipment, recertification may be required by law before clinical use.
-
Buyer is responsible for ensuring the equipment is safe and certified for operational use.
-
We stand by the equipment as described but do not assume responsibility for its use on patients.
Shipping
-
Orders ship from the Instropoint Warehouse once payment is received.
- Worldwide shipment
-
No local pickup is available at this time.
-
Items under 60 lbs ship via FedEx or UPS within the lower 48 U.S. states and DC.
- Anything over 60lbs is shipped Via Freight.
-
If you prefer to use your own shipping account or carrier, we can accommodate that.
Payment
-
We accept credit cards and electronic checks via PayPal.
-
Virginia buyers are subject to 6% sales tax.
-
Sales tax also applies to local pickups (if arranged in the future).
Returns & Refunds
-
Returns due to buyer error or remorse (e.g., ordered by mistake, no longer needed) are subject to:
-
20% restocking fee
-
No refund on original shipping & handling costs
-
-
Shipping charges are non-refundable under all circumstances.
- No refunds for Overseas purchases
- 30 day warranty Warranty on products unless stated otherwise.
Important Regulatory Disclaimer
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration (FDA) and/or state and local regulatory agencies.
If so, do not purchase unless you are an authorized buyer. If required, we will verify your authorization before shipping.
Buyers are solely responsible for ensuring proper certification, inspection, and legal compliance before using any medical device in patient care. We strongly recommend all equipment be inspected by a licensed biomedical technician prior to clinical use.
Still have questions?
We’re here to help — feel free to contact us for clarification.





















Reviews
There are no reviews yet.